Carbon atoms are magical. They can both constitute the world's softest mineral graphite and can also constitute the hardest material diamond in nature. According to the report of the Science and Technology Daily on the 24th, Chinese scientists have recently made breakthroughs in the study of carbon atoms: the birth of a three-dimensional carbon structure T-carbon (T-carbon) predicted by theoretical calculations by Prof. Su Gang of the School of Physics of the Chinese Academy of Sciences. The team of scientists has successfully synthesized T-carbon, making T-carbon an alternative three-dimensional structure that can compete with graphite and diamond. On November 23, Su Gang said in an interview with a reporter from Science and Technology Daily that T-carbon is a fluffy carbon material with a lot of available space inside. If it is used as a material for energy storage, the weight percentage of hydrogen storage capacity is not Less than 7.7%. Su Gang believes that T-carbon will have a wide range of application prospects in fields such as photocatalysis, adsorption, energy storage, and aerospace materials. Since the 1980s, scientists have had a keen interest in acquiring new carbon structures and have spawned two Nobel Prizes. This not only has a huge impact in the related fields such as chemistry, physics, materials, and information science, but also has spawned a large number of industrial and technological applications. Based on these structures, scientists have synthesized many new derivatives and made new functional devices and related products. Carbon atoms have four valence electrons and undergo orbital hybridization (some of the original atomic orbitals are recombined into new atomic orbitals). It is like four hands, and it has a strong combination of itself and other elements. ability. Carbon can form sp2 hybrid graphene, sp3 hybrid diamond, sp-sp2 hybrid graphyne, sp-sp3 hybrid diamond alkynes. "Chemistry, carbon can be combined with other elements, including DNA, proteins and other important biological macromolecules, so that carbon is one of the most basic elements of life on Earth," said Su Gang. In 2011, Su Gang directed doctoral student Sheng Xianlei and others through a large number of comparative studies and proposed that if every carbon atom in cubic diamond is replaced by a tetrahedral structural unit consisting of four carbon atoms, carbon will be formed. A new type of three-dimensional cubic crystal structure. Based on the first-principles study of density functionals, they found that the structure is extremely stable in terms of geometry, energy, and dynamics. They named the new allotrope of carbon as T-carbon. Studies have shown that T-carbon has the same space group as diamond and is a semiconductor with a direct bandgap, which can be used to regulate the bandgap by doping to be suitable for photocatalysis. T-Carbon also has a distinctive feature. The density is very small, about 2/3 of graphite and half of diamond. Su Gang et al. calculated that T-carbon may be more easily formed in a negative pressure environment. T-carbon may be observed in interstellar dust or in extrasolar planets. For the discovery of T-carbon work, industry experts spoke highly of it, saying that "T-carbon has opened a new era in carbon structure research and will inspire other scientists to conduct extensive theoretical and experimental research." Can T-carbon be synthesized in the laboratory? Su Gang has been working hard to promote the experimental synthesis of T-carbon in recent years. In early 2017, the joint research team of Xi'an Jiaotong University and Nanyang Technological University of Singapore succeeded in realizing sp2 from multi-walled carbon nanotubes suspended in methanol solution by picosecond laser irradiation under extreme thermodynamic equilibrium conditions. The transition to the sp3 chemical bond, the formation of a new carbon material is exactly the same as the theoretical prediction of T-carbon, demonstrating the synthesis of T-carbon. The results of the experiment on the synthesis of T-carbon were announced in Nature News shortly before.
Melamine Particle Board is mainly used for furniture and carriage of bus, train.The bace&back are the Veneer surface, such as Okoume, Bintangor, Pine, Birch, Poplar, Pencil cedar, Maple, Cherry, White Oak, Sapele, Beech, Red Oak, Ash etc.Melamine paper is the most popular to be as the face board, it is abrasion resistant, heat resistant, fouling resistant, clean is simple.The Engineering wood is also as the face and back surface. It is more cheaper and beautiful, can reach the same wood grain effect as the veneer surface.And we have High Quality Particle board .LULI Group Co. Ltd, well known as the leading manufacturer for wooden, steel and paper products, located in Shouguang, Weifang, Shandong, China. Since the foundation in 1985, it focus on the production of Plywood , venner, MDF , Particle Board , Door skin , Blockboard , Finger Joint Board, OSB , paper, Steel etc.And we have Outdoor Melamine Particle Board.
Melamine Particle Board Details:
size:1220*2440MM 1250*2000MM 1525*2440MM 1830*2440MM
THICKNESS:9MM-40MM
MATERIAL:POPLAR, COMBINE, PINE
GLUE:E0, E1, E2
CERTIFICATION:CARB, FSC, CE
Melamine Particle Board Melamine Particle Board,Melamine Faced Particle Board,Outdoor Melamine Particle Board,Melamine Laminated Particle Board Luli Group Co.,Ltd. , https://www.cnluli.com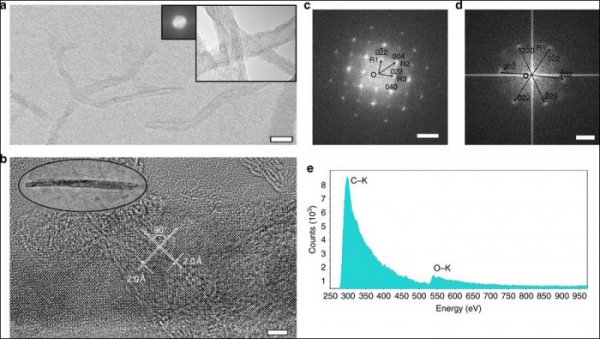
Structural Transformation of MWCNTs (Multiwalled Carbon Nanotubes) to T-Carbon Nanowires 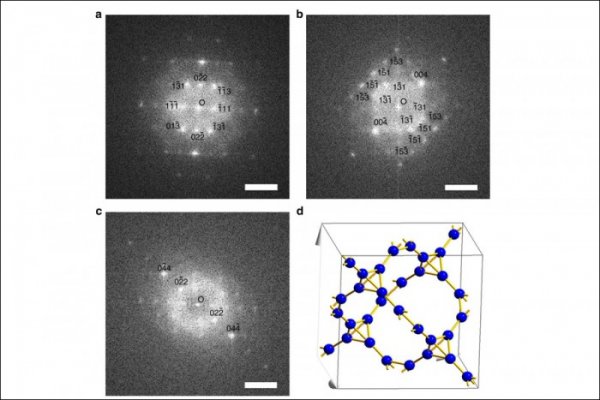
FFT Patterns and Structural Models of Single T-Carbon Nanowires at Different Angles